What is Zivek?
Zivek contains Imatinib (as mesylate) as the active ingredient. Imatinib is a white or off-white to brownish crystalline powder.

Zivek mechanism of action
Imatinib is a protein-tyrosine kinase inhibitor used in therapy of cancers.
Indications and usage
Imatinib is used in:
- acute lymphoblastic leukemia (ALL)
- chronic myeloid leukemia (CML)
- Chronic Eosinophilic Leukemia or hypereosinophilic syndrome (CEL/HES)
- myelodisplasia or myeloproliferative diseases (MDS/MPD)
- gastrointestinal stromal tumors (GIST)
- aggressive systemic mastocytosis (ASM)
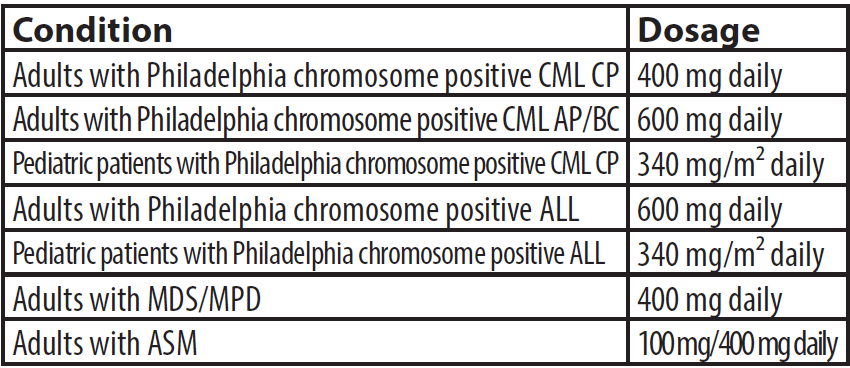
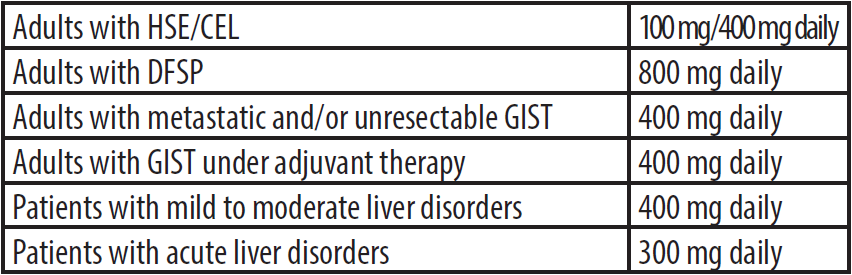
Dosage and administration
Zivek should be swallowed with a meal and a large glass of water. Doses of 400 mg and 600 mg are taken once daily and 800mg dose is taken in 400mg doses twice daily. The tablet may be dispersed in a glass of water or apple juice in individuals unable to swallow and should be taken immediately after complete disintegration of tablet(s). First, in order to observe the safety principles in preparation of oral solution, the person
should use gloves and apron to prevent contamination. Each 100 mg of imatinib should be mixed in 50 ml of water or apple juice, for example, imatinib 400mg in 200 ml of water or apple juice. The required number of tablets should added slowly into the still water or apple juice and stir well with a spoon until completely dissolved. Ensure the patient drinks the whole contents of the glass immediately. In the end, place the used gloves in a special plastic bag for hazardous drugs.
Dosage forms and strengths
Imatinib (as mesylate) is available in scored film-coated tablets. It comes in 100 mg and 400 mg strengths.
Warnings and precautions
Edema and fluid retention
Patients taking Imatinib usually experience edema, fluid retention and weight gain. Patients’ weight has to be regularly checked and in case of any unexpected rapid weight gain or any other signs and symptoms indicating fluid retention, necessary therapy measurements should be taken. In patients with cardiovascular diseases (heart dysfunction and/or high blood pressure) and lung diseases when fluid retention is barely tolerable, Imatinib should be used with caution.
Bone marrow suppression
Bone marrow suppression in the forms of cytopenia, anemia, neutropenia and thrombocytopenia can occur while taking Imatinib. To this effect, your doctor may order blood cells count every week during the first month and every two weeks during the second month of therapy and periodically afterwards based on clinical symptoms.
Congestive heart failure and left ventricular dysfunction
Severe heart failure and left ventricular dysfunction have been reported in patients and particularly in patients with pre-existing diseases and/ or patients with risk factors for cardiovascular diseases. To this effect, in patients with heart problems and/or with risk factors for heart dysfunction Imatinib should be taken with caution and heart function be monitored while under therapy.
Liver toxicity
Cases of severe liver dysfunction in patients receiving Imatinib or in combination with chemotherapy regimens have been reported. Therefore, both before and after taking Imatinib the patient liver function should be monitored every month. In case of emerging liver problems, discontinuing therapy or reducing the dose may take place.
Bleeding
Class III and IV bleeding have been observed in clinical studies in newly diagnosed patients with CML and GIST. Gastrointestinal tumors can cause bleeding in GIST. Gastrointestinal symptoms should be monitored before taking Imatinib.
Gastrointestinal disorders
Gastrointestinal perforation that may be fatal has also been reported in some patients receiving Imatinib. To this effect, Imatinib should be taken with a meal and a large glass of water.
Skin disorders
Skin reactions like erythema multiform and Stevens-Johnson syndrome
have been reported in patients. In case of such reactions, stop using Imatinib
and do not use it again.
Hypothyroidism
Clinical cases of hypothyroidism have been reported in thyroidectomy patients undergoing levothyroxine replacement. Monitoring TSH levels and modifying doses of thyroid (hormone) supplements is recommended while in therapy with Imatinib.
Embryo-Fetal toxicity
Fetal toxicity may occur in pregnant women receiving Imatinib. Due to possible hazards, women must avoid pregnancy.
Growth retardation in children and pre-adolescents
Reports of growth retardation in children and pre-adolescents have been made. To this effect, their growth should be closely monitored while taking Imatinib.
Tumor lysis syndrome
This syndrome can be potentially hazardous and has been reported in patients with ALL, CML, GIST and eosinophilic leukemia. Correcting dehydration and treating high uric acid levels prior to initiation of Imatinib can help prevent the syndrome.
Impairments related to driving and operating machinery
Patients receiving Imatinib must be cautioned about driving and/or operating machinery that need accuracy and vigilance. Incidences of dizziness, blurred vision or drowsiness may occur in patients receiving Imatinib.
Adverse effects
Common side effects
-cardiovascular disorders: edema, hypotension, chest pain nervous system/psychiatric disorders: fatigue, headache, depression, anxiety, taste disturbances, insomnia, muscle cramps
-skin disorders: skin rash, itching (pruritus), night sweats, hair fall
-gastrointestinal disorders: nausea, diarrhea, vomiting, poor appetite, abdominal pain, bloating, constipation, dry mucus
-blood disorders: low levels of white and red blood cells and platelets, bleeding
-liver disorders: changes in liver enzymes levels, rising levels of bilirubin In case of any adverse effects while taking Imatinib, contact your doctor or therapy team.
Zivek drug interactions
To best manage such interactions, be sure to inform your doctor of any medication you use before starting Imatinib.
-Imatinib may interact with vaccines and medicines such as warfarin, erythromycin, phenytoin and even herbal products, St. John’s Wort, grapefruit juice, etc.
-While taking Imatinib, avoid using medication and food supplements that inhibit or induce the effects of CYP3A4.
Use in specific populations
Pregnancy
Imatinib is in D pregnancy risk (letter) category. Based on animal and human data available, Imatinib can cause fetal toxicity. Therefore, advise patients to avoid pregnancy when taking Imatinib and 14 days after the last dose.
Nursing
Studies indicate that Imatinib is excreted into nursing mother milk and can cause serious adverse effects in new born. To this effect, avoid nursing when taking imatinib and 1 month after the last dose.
Pediatric use
Immunity and efficacy of Imatinib in children with (Ph+) CML and (Ph+) ALL has been proved. However, there are no data in children under 1 year of age.
Geriatric use
According to studies, efficacy of Imatinib in this group is the same as other individuals. Studies, however, indicate a higher incidence of edema in this group. As for other adverse effects, no obvious differences were noted.
Liver disorders
As acute liver impairment may affect Imatinib absorption, it is recommended to reduce the dose by 25% as compared to other patients.
Kidney disorders
Studies indicate that Imatinib in patients with renal impairment (kidney disorders) may alter drug consistency and absorption. Therefore, dose reductions are certainly necessary for these patients.
Overdose
In case of Imatinib overdose, make sure to contact your doctor or healthcare provider.
Missed dose
In case you forget taking your dose, immediately take it as soon as you remember. Do not take the missed dose if it is almost the time to take the next dose and take your daily dose according to the given schedule.
Zivek storage
Keep away from light and moisture, Store below 30˚C.
Zivek composition
Active ingredient: Imatinib (as mesylate) Inactive ingredients: colloidal silicon dioxide (NF); crospovidone (NF); hydroxypropyl methylcellulose (USP); magnesium stearate (NF); and microcrystalline cellulose (NF). Tablet coating: ferric oxide, red (NF); ferric oxide, yellow (NF); hydroxypropyl methylcellulose (USP); polyethylene glycol (NF) and talc (USP).
D.Nurse-Patient Helpline Team
You can contact Drugs Expert Consultation Center at 0935 220 3041-4 to get answers to your inquiries and also get to know nursing support centers in your city.Expert Consultation Center is always ready to answer the questions of our valued customers.



